Comparing Animal and Plant Cell Structures through Coloring: Animal And Plant Cell Coloring
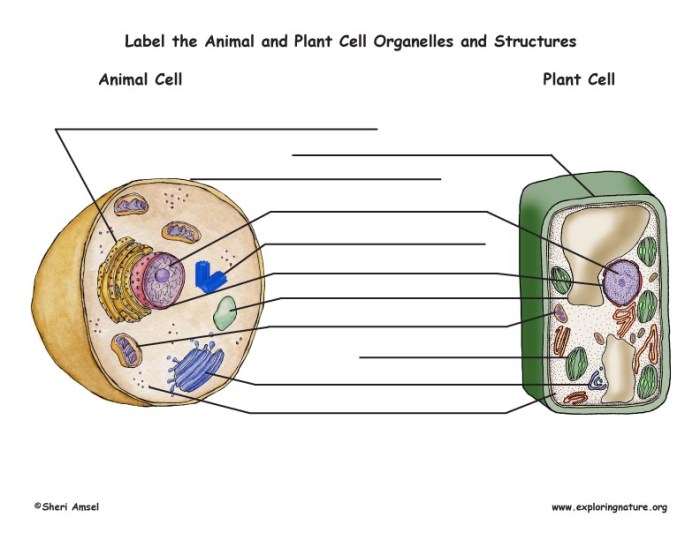
Animal and plant cell coloring – Coloring exercises provide a valuable hands-on approach to understanding the fundamental differences between animal and plant cells. By visually distinguishing various cellular components through differential staining, students can gain a deeper appreciation for the unique structures and functions of each cell type. This method allows for a direct comparison of key features, solidifying theoretical knowledge with practical observation.
Basic Structural Differences, Animal and plant cell coloring
Animal and plant cells share some common features, including a nucleus, cytoplasm, mitochondria, and a cell membrane. However, significant differences exist. Plant cells possess a rigid cell wall composed primarily of cellulose, providing structural support and protection. This contrasts sharply with animal cells, which lack a cell wall and rely on their flexible cell membrane for maintaining shape. Furthermore, plant cells typically contain chloroplasts, the organelles responsible for photosynthesis, which are absent in animal cells.
The presence or absence of these structures is readily apparent through staining techniques.
Staining Techniques and Cellular Component Interactions
Various stains are employed to highlight specific cellular components. For example, methylene blue, a common stain, readily binds to negatively charged components within the cell, such as nucleic acids in the nucleus, resulting in a dark blue coloration. Iodine, on the other hand, stains starch granules within the chloroplasts of plant cells a deep purple or black, clearly distinguishing these organelles.
The cell wall, being relatively inert, may not stain intensely with many dyes, but its presence is clearly evident as a distinct outer boundary. The differential staining highlights the structural differences between the cells, emphasizing the presence of a cell wall in plant cells and the absence of chloroplasts in animal cells.
Comparison of Common Stains
The following table summarizes the interaction of four common stains with various cellular structures in animal and plant cells:
| Stain | Target Structure | Animal Cell Result | Plant Cell Result |
|---|---|---|---|
| Methylene Blue | Nucleus, Cytoplasm | Dark blue nucleus and stained cytoplasm | Dark blue nucleus and stained cytoplasm; cell wall may show minimal staining |
| Iodine | Starch Granules | No significant staining | Deep purple/black staining of starch granules in chloroplasts |
| Crystal Violet | Cell Wall (in conjunction with other stains) | No staining of cell wall | Dark purple staining of the cell wall |
| Acetocarmine | Chromosomes (during cell division) | Deep red staining of chromosomes | Deep red staining of chromosomes |
Specific Staining Techniques and Procedures
Staining techniques are crucial for visualizing the intricate structures of both plant and animal cells, which are otherwise largely transparent under a light microscope. Different stains bind to specific cellular components, enhancing contrast and revealing details about cell morphology and organization. This section will explore specific staining methods, focusing on their application to both plant and animal cells, and provide detailed protocols for slide preparation.
Gram Staining Procedure for Bacterial Cells and Adaptations for Eukaryotic Cells
Gram staining is a differential staining technique primarily used to distinguish between Gram-positive and Gram-negative bacteria based on differences in their cell wall composition. While not directly applicable to plant or animal cells in the same way, understanding the principle can inform other staining approaches. The procedure involves four steps: 1) applying crystal violet (primary stain), 2) adding Gram’s iodine (mordant), 3) decolorizing with alcohol or acetone, and 4) counterstaining with safranin.
Gram-positive bacteria retain the crystal violet-iodine complex and appear purple, while Gram-negative bacteria lose the complex and are stained pink by the safranin. Adapting this for eukaryotic cells would involve choosing stains that target specific organelles or cellular structures rather than relying on cell wall differences. For example, a similar sequential staining approach could be used with different dyes targeting the nucleus (e.g., hematoxylin) and cytoplasm (e.g., eosin) to differentiate these compartments in animal cells.
Methylene Blue Staining in Animal and Plant Cell Observation
Methylene blue is a basic dye that stains acidic components of cells, such as nucleic acids and some proteins, a dark blue color. It’s a simple and widely used stain for observing both plant and animal cells. In animal cells, it highlights the nucleus and cytoplasm, revealing overall cell shape and size. In plant cells, methylene blue stains the nucleus and can also highlight the cell wall, though it may not penetrate the cell wall as effectively as other stains.
The simplicity of methylene blue staining makes it an excellent introductory stain for microscopy.
Protocol for Preparing Slides for Animal and Plant Cell Observation Using Methylene Blue
This protocol details the preparation of slides for observing animal and plant cells using methylene blue staining.
- Materials: Microscope slides, coverslips, methylene blue stain (0.5% aqueous solution), distilled water, tweezers, scalpel or razor blade, blotting paper, light microscope, onion bulb (for plant cells), cheek swab (for animal cells).
- Procedure (Plant Cells):
- Peel a thin layer of epidermis from the inner surface of an onion bulb using tweezers.
- Place the epidermis onto a clean microscope slide.
- Add a few drops of 0.5% methylene blue stain to the epidermis.
- Gently add a coverslip, avoiding air bubbles.
- Blot away excess stain using blotting paper.
- Observe under a light microscope.
- Procedure (Animal Cells):
- Gently scrape the inside of your cheek with a clean cotton swab.
- Smear the swab onto a clean microscope slide, creating a thin layer.
- Allow the smear to air dry completely.
- Add a few drops of 0.5% methylene blue stain to the smear.
- Gently add a coverslip, avoiding air bubbles.
- Blot away excess stain using blotting paper.
- Observe under a light microscope.
Preparation of a 0.5% Methylene Blue Working Solution
To prepare a 0.5% aqueous solution of methylene blue, dissolve 0.5 grams of methylene blue powder in 100 milliliters of distilled water. Stir gently until the powder is completely dissolved.
Safety Precautions: Methylene blue can stain skin and clothing. Wear gloves and eye protection during preparation and handling. Avoid inhalation of the powder. Dispose of used solutions properly according to local regulations.
Microscopic Observation and Interpretation of Stained Cells
Successful microscopic observation of stained cells relies on understanding the principles of light microscopy and employing proper techniques. Clear, high-quality images are crucial for accurate interpretation, allowing for the precise identification of cellular structures and the detection of any artifacts that may skew results. This section details the necessary procedures and interpretation guidelines.
Understanding the differences between animal and plant cell coloring can be a fun and educational activity. For instance, you might consider supplementing your cell studies with a visual aid like the charming animal alphabet coloring sheets , which helps illustrate basic animal anatomy. This approach can then be extended to enhance comprehension of the structures within animal cells, and how they differ from those in plant cells.
Principles of Microscopy for Stained Cell Observation
Light microscopy, specifically brightfield microscopy, is the most common technique used to visualize stained cells. The process involves illuminating the sample with light, which passes through the specimen and is then magnified by a series of lenses. Staining enhances contrast, making cellular components more visible. The resolution of the microscope, determined by the wavelength of light and the numerical aperture of the lenses, dictates the smallest distance between two points that can be distinguished as separate entities.
Higher numerical apertures and shorter wavelengths lead to better resolution. Proper adjustment of the condenser and focus is essential for optimal image quality. Understanding the magnification levels and the depth of field (the range of distances within the sample that appears in sharp focus) are also critical for accurate observation.
Best Practices for Obtaining High-Quality Microscopic Images
Several practices contribute to obtaining clear and high-quality images of stained cells. First, ensure the microscope is properly cleaned and aligned. Clean the lenses using lens paper and appropriate cleaning solutions. Then, prepare a high-quality, thin sample mount to minimize light scattering and ensure even illumination. Using a coverslip improves flatness and prevents damage to the objective lens.
Next, adjust the condenser to optimize light intensity and focus. The condenser should be raised to its highest point for maximum resolution. Begin focusing at low magnification to locate the specimen and then gradually increase the magnification while carefully adjusting the fine focus. Use proper lighting techniques, avoiding overexposure which can wash out detail or underexposure that obscures structures.
Finally, consider using immersion oil with high-power objectives (e.g., 100x) to improve resolution and light transmission.
Interpreting Staining Results in Animal and Plant Cells
Different staining techniques reveal different cellular structures. For example, hematoxylin and eosin (H&E) staining, a common technique in histology, stains cell nuclei purple (hematoxylin) and cytoplasm pink (eosin). In animal cells, this allows for the visualization of the nucleus, cytoplasm, and potentially other organelles depending on the cell type and staining protocol. In plant cells, H&E staining highlights the nucleus and cytoplasm similarly, but the cell wall remains visible, often appearing as a clear Artikel around the cell.
Other stains, such as iodine (for starch granules) or methylene blue (for general cell structures), provide additional information specific to the targeted cellular component. Careful comparison of stained cells from both animal and plant sources allows for a thorough understanding of their structural differences.
Identifying Artifacts in Microscopic Images
Artifacts are structures or features in a microscopic image that are not actually part of the biological sample. They can arise from various sources, including improper sample preparation (e.g., air bubbles, debris), staining issues (e.g., uneven staining, precipitate formation), or problems with the microscope itself (e.g., scratches on the lens). Recognizing artifacts is crucial to avoid misinterpreting them as real cellular components.
Common artifacts include air bubbles (appearing as clear, circular spaces), dust particles (appearing as dark spots), and precipitates from the stain (appearing as irregular clumps). Careful observation and comparison with known cellular structures can help distinguish artifacts from genuine cellular features. Consistent and meticulous sample preparation techniques minimize the risk of artifact introduction.
Applications of Cell Coloring in Biological Research
Cell staining, a cornerstone of biological research, significantly enhances our ability to visualize and analyze cellular structures and processes. These techniques are indispensable across various biological disciplines, providing crucial insights into both normal cellular function and disease mechanisms. The specificity and sensitivity of different stains allow researchers to target particular cellular components, revealing intricate details otherwise invisible with standard microscopy.Cell coloring techniques have revolutionized our understanding of biological systems, impacting diagnosis, treatment strategies, and fundamental research across diverse fields.
The application of these techniques ranges from identifying infectious agents in clinical pathology to unraveling the complex interactions within plant tissues in botany. The appropriate selection and execution of staining protocols are critical for obtaining accurate and reliable results, which directly translate into improved healthcare and advancements in scientific knowledge.
Applications in Pathology
In pathology, cell staining is essential for diagnosing a wide range of diseases. For instance, the Gram stain differentiates bacteria based on their cell wall composition, allowing for rapid identification of bacterial infections and guiding antibiotic treatment. Similarly, the Pap smear, a cytological staining technique, screens for cervical cancer by identifying precancerous and cancerous cells. The accurate interpretation of these stained samples is critical for timely and effective intervention, significantly impacting patient outcomes.
Immunohistochemistry (IHC), which uses antibodies labeled with chromogens to detect specific proteins within cells, is another powerful technique used to diagnose various cancers and other diseases by identifying the presence or absence of particular biomarkers. The results of IHC staining can help determine the type of cancer, its aggressiveness, and the most appropriate treatment strategy.
Applications in Cytology
Cytology relies heavily on cell staining to analyze individual cells and their components. Techniques like hematoxylin and eosin (H&E) staining provide crucial information about cell morphology, enabling the identification of abnormal cells indicative of cancer or other diseases. Specific stains can highlight features such as nuclear morphology, cytoplasmic inclusions, and the presence of certain organelles, providing detailed insights into cellular health and function.
These detailed cellular analyses are crucial in diagnosing various blood disorders, cancers, and infectious diseases. Furthermore, the use of fluorescent stains in flow cytometry allows for the simultaneous analysis of multiple cellular parameters, enabling high-throughput screening and analysis of cell populations.
Applications in Botany
In botany, cell staining plays a vital role in understanding plant structure, function, and development. Staining techniques reveal the intricate details of plant cell walls, chloroplasts, and other organelles, providing insights into processes like photosynthesis, nutrient transport, and cell division. Specific stains can highlight the presence of certain polysaccharides, proteins, or lipids within plant tissues, helping researchers investigate the composition and function of various plant structures.
For example, the use of iodine staining can help visualize starch granules within plant cells, providing information about carbohydrate storage and metabolism. Moreover, the application of fluorescent dyes allows for live-cell imaging, enabling researchers to observe dynamic processes within plant cells without causing cell death. This capability is particularly useful for studying plant responses to environmental stresses.
Common Queries
What safety precautions should be taken when handling stains?
Always wear appropriate personal protective equipment (PPE), including gloves and eye protection. Work in a well-ventilated area and follow the specific safety guidelines provided by the stain manufacturer.
How do I clean the microscope slides after staining?
Gently rinse the slides with distilled water, then carefully wipe them clean with lens paper. Avoid scratching the slides.
What are some common artifacts that can be observed in stained cell preparations?
Common artifacts include air bubbles, debris, and uneven staining. Proper slide preparation techniques can minimize these artifacts.
Can I use the same staining technique for all types of plant and animal cells?
No, different cell types may require different staining techniques to optimally visualize their structures. The choice of stain depends on the specific cellular components of interest.
